klmn shell and spdf|What is the difference between SPDF and KLMN shell? : Baguio Its a fact that each shell itself is composed of subshells (experiments involving .
Ibabang Dupay, Red -V Lucena City Courtesy/Credits by: Catryona Lei https://www.tiktok.com If your TikTok is in this compilation and you want it to be removed, please e-mail barcelosdiether0@gmail .
PH0 · Where is SPDF orbitals?
PH1 · What is the difference between the KLMN and SPDF methods of
PH2 · What is the difference between SPDF and KLMN shell?
PH3 · What is the difference between KLMN orbits and SPDF orbits of ..
PH4 · What is SPDF configuration?
PH5 · What is SPDF and KLMN?
PH6 · Electronic configuration
PH7 · Electron Configuration Chart of All Elements (Full Chart)
PH8 · Electron Configuration Chart
PH9 · Electron Configuration
PH10 · 2.4 Electron Configurations
About SpamCalls.net Search for Phone Numbers and identify unwanted Calls. Recognize Spam Calls (Ping Calls, Robocalls) with the phone number reverse search / caller id lookup. Stop unwanted calls and get the caller id for an unknown number. emoji_emotionsBy and for people like you and me! emoji_emotions100% true reports!
klmn shell and spdf*******What is the difference between the KLMN and SPDF methods of finding
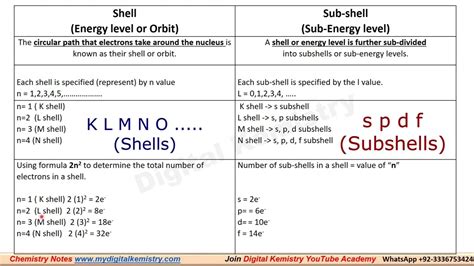
Electron Configuration - Definition, Uses and Solved ExamplesElectron Configuration - Definition, Uses and Solved ExamplesWhat is the difference between the KLMN and SPDF methods of findingK denotes the first shell (or energy level), L the second shell, M, the third shell, and so on. In other words, the KLMN(OP) notation only indicates the number of electrons an atom has .Its a fact that each shell itself is composed of subshells (experiments involving .
this tutorial video will teach you everything you need to know about arranging electrons in K,L,M,N SHELL and Spdf orbital of an atom, from scratch to finis.
Set 4, 2022 — The difference between the KLMN and SPDF is K denotes the first shell or energy level, L the second shell, M, the third shell, and so on. In other words, the KLMN .klmn shell and spdfIts a fact that each shell itself is composed of subshells (experiments involving spectra have shown this). The number of subshells each shell has depends on the number of .Mar 23, 2023 — Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Free Gift for you: Interactive Periodic Table. Let me tell you how this Interactive Periodic Table will help you in your studies. 1).spdf Notation. The most common way to describe electron configurations is to write distributions in the spdf notation. Although the distributions of electrons in each orbital are not as apparent as in the diagram, the total .107 rows — Peb 1, 2021 — The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state . This handy chart compiles the electron configurations of .
Set 2, 2022 — What is KLMN and SPDF? The difference between the KLMN and SPDF is K denotes the first shell or energy level, L the second shell, M, the third shell, and so on. In other words, the KLMN notation only indicates the number of electrons an atom has with each principal quantum number.
May 28, 2023 — Students who ask this question also asked. CHEMICAL BONDING AND MOLECULAR STRUCTURE 109 and Powell in 1940, proposed a simple theory result in deviations from idealised shapes and based on the repulsive interactions of the alterations in bond angles in molecules. electron pairs in the valence shell of the !KLMN shells are labels used to represent different energy levels or shells in the electron configuration. The K shell corresponds to the first energy level, the L shell to the second .For example, the names of the subshells in a sulfur atom would be 1s, 2s, 2p, 3s, and 3p (since sulfur has three electron shells). All of these shells are filled except the 3p shell which has four electrons. Therefore, the .Okt 7, 2023 — Welcome to our blog post on the topic of converting liters per minute (LPM) to pounds per square.

Pauli Exclusion Principle. The Pauli exclusion principle states that no two electrons can have the same four quantum numbers. The first three (n, l, and m l) may be the same, but the fourth quantum number must be .In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom's nucleus.The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (or "L shell"), then the "3 shell" (or "M shell"), and so on farther and farther from the nucleus.klmn shell and spdf What is the difference between SPDF and KLMN shell? Shells. An electron shell may be thought of as an orbit followed by electrons around an atom nucleus. Because each shell can contain only a fixed number of electrons, each shell is associated with a particular range of electron energy, and thus each shell must fill completely before electrons can be added to an outer shell.Okt 21, 2021 — Define Shell and Subshell in Chemistry | klmn Shell in ChemistryIn this video we will come to know Shell | Sub Shells | KLMN Shells | spdf sub shell | Energy.What are klmn shells? What is Spdf in periodic table? Why is 3rd shell 8 or 18? What is K shell called? What is the 2 8 8 18 rule in chemistry? Introduction. Electronic configuration refers to the arrangement of electrons within an atom or a molecule. It provides information about how electrons are distributed among different energy levels .Electron Configuration -The Electron Configuration of an Element Describes how Electrons are Distributed in their Atomic Orbitals. In Electronic Configuration electrons are arranged in various shells, Subshell and Orbital by following certain rules. To Learn how to Write Electronic Configurations, Detailed Explanation, Filling of orbital with FAQs, Visit .What is the difference between SPDF and KLMN shell? Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.Set 5, 2022 — Spdf or SPDF may refer to: Electron configuration, for which there is an obsolete system of categorizing spectral lines as “sharp”, “principal”, “diffuse” and “fundamental”; also the names of the sub shells or orbitals.Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.As it turns out, the K type X-ray is the highest energy X-ray an atom can emit. It is produced when an electron in the innermost shell is knocked free and then recaptured. This innermost shell is now called the K-shell, after the label used for the X-ray. Barkla won the 1917 Nobel Prize for Physics for this work.Mar 14, 2012 — K,L,M,N电子层和spdf之间的关系!K能层有1S能级L能层有2S、2P能级M能层有能级N能层有4S、4P、4d、4f 能级s能级有 1个轨道 最都容纳 .A shell is the path that electrons take around the nucleus of an atom. These shells are also known as energy levels because they are grouped around the nucleus according to the energy of an electron in each shell. The nucleus is closest to the shell with the lowest energy. Beyond that shell, the next energy level can be found.Shell - Electron- Electrons revolve around the nucleus in a specific circular path known as orbit or called shell. Shells have stationary energy levels, the energy of each shell is constant. To learn more about the Character, Definition, subshell, energy of subshell, Arrangement of electrons in shell with FAQs, Visit BYJU’S
The maximum number of electrons that can be accommodated in any shell according to Bohr and bury is 2 × n 2, where n is the shell number K-Shell will be= 2 × 1 2 = 2 L- Shell will be= 2 × 2 2 = 8
Peb 20, 2014 — The first shell can carry up to two electrons, the second shell can carry up to eight electrons. The third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. There is a formula for obtaining the maximum number of electrons for each shell which is given by $2n^2~\ldots$ where n is the position of a .
Hun 28, 2022 — In each of the subshells s, p, d, and f, the maximum number of electrons allowed is 2, 6, 10, and 14 accordingly. Noble gases, which have entirely filled outermost shells and can be prefixed to the outer shell of the element, can also be used to write the electronic configuration of elements, and the electronic configuration must be noted.
Sie möchten Ihr/e Los/e bei der BOESCHE KG zu kündigen? Jetzt zum Online-Formular » . Wollen Sie wirklich mit einer Kündigung auf alle Gewinnchancen verzichten? . im Verlauf der Lotterie mit dem NKL Extra-Joker eine 5.000-€-Rente zu gewinnen, beträgt 1 : 1.666.667. Das maximale Verlustrisiko ist der Spieleinsatz.
klmn shell and spdf|What is the difference between SPDF and KLMN shell?